On 9th and 10th May 2019, PVpharm provided a pharmacovigilance training in Madrid.

We had the honour to have as trainers:
- Mariano Madruga: a well known professional with more than 30 years dedicated experience in pharmacovigilance, being an official of the Ministry of health/AEMPS and developing the pharmacovigilance Network in Spain.
- Calin Lungu: Dr. Lungu has worked more than 20 years in drug development, clinical research, pharmacovigilance and quality assurance. He has done more than 140 PV audits and trained more than 250 Eudravigilance and XEVMPD courses at the EMA
- Albert García Rierola: The EU-Qualified person responsible for pharmacovigilance (QPPV) at Ferrer International. He has experience in different areas such as regulatory affairs and price and reimbursement,
- Maite Vázquez: economist and lawyer, with training in Biotechnology and Pharma Law. She is the secretary of the Board of the Spanish Biotechnology Association (ASEBIO) and legal advisor in the Pharma and Health group of the Bar Association of Madrid (ICAM) and she has been advising companies in pharma and bio industry in regulatory, contracts, corporate and compliance matters.
- José Alberto Ayala Ortiz: Pharmacist with specialty in IT who has been working more than 16 years in Pharmacovigilance and Drug Safety in multiple places including the Danish Agency and also as a trainer for EVWEB and XEVMPD trainings at the EMA and other locations.
During this course, we focused on different aspects and areas such as how a PV department is organized, the role of the QPPV and how this function is audited, personal data protection issues in PV, latest updates in the use of EVDAS for signal detection, ICSRs, PSURs, etc.
The training was held in Madrid, at the emblematic Hotel Emperador. The course was held in English, and there were participants coming from several locations across Europe. We could enjoy a fantastic atmosphere during the training and we would like to thank the participants for their valuable and professional contribution to the training.
Just like in the 1st edition, after the training, an anonymous questionnaire was administered to the participants in order to assess their satisfaction and to improve our performance on the coming editions. We would like to highlight 2 questions form the reviews:
Question 1. This course was…
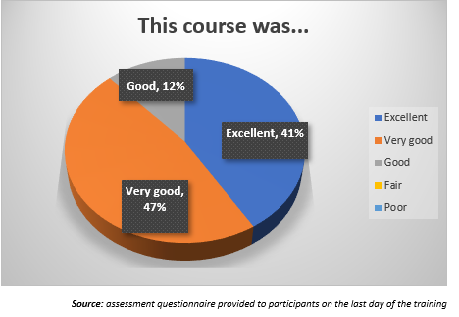
As we can see, most of the participants, around 88%, describe the course as “excellent” or “very good”.
Question 2: How likely would you recommend this course to a friend or colleague (from 0 “not likely at all” to 10 “extremely likely”.
Participants assess the possibility of recommending this course to a friend or colleague as 9 (mean of the total answers).
The results were quite positive and we are very delighted and thankful to everyone for their inputs. We are looking forward to the 3rd edition!
PVpharm team.




